Design strategy for nanoplatform Cy-NMNO@SiO2
Scheme 1a outlines the synthesis of nanoplatform Cy-NMNO@SiO2. All synthesized compounds were confirmed by 1H NMR, 13C NMR and liquid chromatography ion trap mass spectrometry (LC-MS). The synthesis details of the nanoplatforms are shown in the Supporting Information. Typically, hypoxia is one of the major therapeutic barriers to PDT, whereas hyperoxia promotes ROS production and enhances the killing effect on tumors and bacteria. Therefore, we first designed a fluorescent probe, Cy-NMNO, which can release both NO and photosensitizer under near-infrared laser irradiation for the treatment of tumors and antibacterials and to ameliorate the hypoxia problem in photodynamic therapy (Scheme 1b). In this scheme, we chose a benzoheptamethine cyanine dye with a benzo-indole heterocycle as a photosensitizer, which is due to the fact that when the heterocycle in the structure of the benzoheptamethine cyanine dye is changed from an indole heterocycle to a benzo-indole heterocycle, the nature of the push-pull electronic structure of the a benzoheptamethine cyanine dye is changed. This affects the conjugated nature of the phorbol ester system, resulting in a red shift in the absorption and fluorescence spectra, which gives it a longer near-infrared excitation wavelength and improves the shortcomings of the traditional laser light scattering. And it can penetrate deeper tissues and thus excite more photosensitizer-enriched regions. We then combined the nitrosamine group with the fluorophore Cy to obtain the compound Cy-NMNO with a high molar absorption coefficient and near-infrared (NIR) emission.
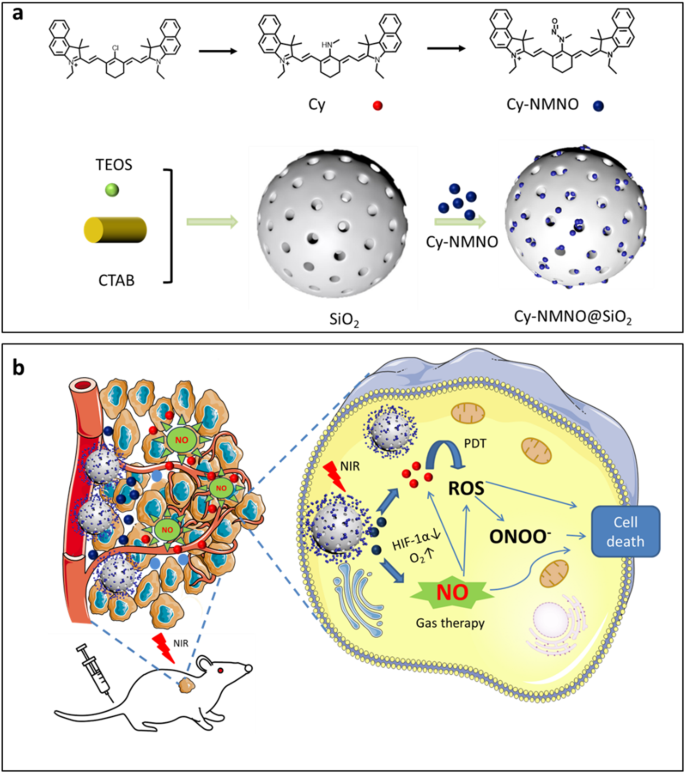
(a) Schematic illustration of the synthesis route of Cy-NMNO@SiO2. (b) The mechanism of the Cy-NMNO@SiO2 for the PDT/GT
In addition, MSN particle size controls cellular interactions as well as distribution and elimination. It has been reported in the literature that the optimal MSN size to promote accumulation at the tumor site and provide a longer circulating half-life is in the range of 50–300 nm. Smaller MSNs (less than 50 nm) have poor porosity, while larger MSNs (greater than 300 nm) have impaired diffusion into the tumor mesenchyme [24]. Therefore, we synthesized MSNs with sizes around 50–100 nm for loading small molecule photosensitizers.
Characterization of Cy-NMNO@SiO2
Cy-NMNO@SiO2 was then characterized in detail. Transmission electron microscopy (TEM) imaging and DLS analysis showed that the size of Cy-NMNO@SiO2 was about 50–100 nm with good particle homogeneity (Fig. 1a and b). The spectral properties of Cy@SiO2, Cy-NMNO@SiO2 were investigated under simulated physiological conditions (10 mM HEPES, pH 7.4). As shown in Fig. 1c, Cy@SiO2 has a strong absorption around 800 nm, which is favorable for its application in NIR laser-based photodynamic therapy. After substitution and nitration of the exposed amino group, the electron-donating ability of the amino group is greatly reduced, leading to the PET process accompanied by fluorescence burst. We hypothesized that under the excitation of 808 nm light, the uniform breakage of the N-N bond leads to the departure of the substituent and the fluorescence turns on. To verify the role of Cy-NMNO@SiO2 in response to NIR laser light, we divided the Cy-NMNO@SiO2 solution into two groups. The fluorescence activation behavior in the NIR was verified by fluorescence spectroscopy. As shown in Fig. 1d, the fluorescence emission intensity of Cy-NMNO@SiO2 solution after NIR laser irradiation was significantly enhanced, reaching the maximum emission wavelength at 810 nm. Both the maximum absorption wavelength and fluorescence emission wavelength were located in the near-infrared region. Energy dispersive X-ray spectroscopy (EDS) elemental mapping (Fig. 1e) showed that Cy-NMNO is uniformly distributed in mesoporous silica.
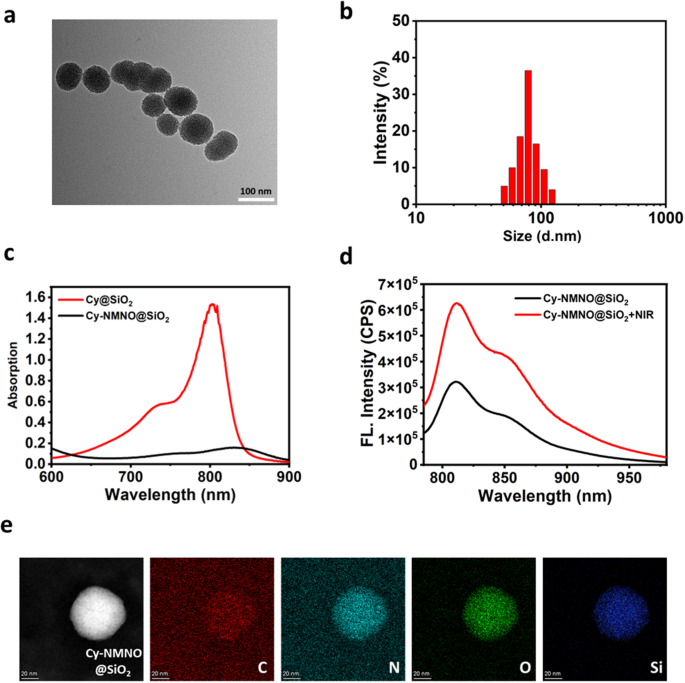
(a) A representative TEM image for Cy-NMNO@SiO2. (b) DLS data of the Cy-NMNO@SiO2. (c) Absorption spectra of Cy@SiO2, Cy-NMNO@SiO2. (d) After laser irradiation (5 min, 808 nm), the fluorescence emission spectrum of Cy-NMNO@SiO2 changed. All data were collected in HEPES (pH 7.4) at 37 ℃ (λex = 730 nm, λem = 810 nm). (e) EDS elemental mapping of Cy-NMNO@SiO2. Scale bar = 20 nm
Study on the activation and release of Cy-NMNO@SiO2 in vitro
To demonstrate that our synthesized nanoplatforms have the function of activation under NIR light irradiation, we imaged the live cells with confocal laser scanning microscopy (CLSM) to observe the activation process. Human malignant melanoma cells (A375 cells) were treated with Cy-NMNO@SiO2 (6 µg/mL) for 2 h, washed, and subjected to NIR laser irradiation (808 nm, 5 min, 1.5 W cm-2). The cells were then washed three times with Dulbecco’s modified eagle medium (DMEM) to remove excess Cy-NMNO@SiO2. As shown in Fig. 2a, there was almost no fluorescence in the cell group irradiated by NIR laser alone with the addition of Cy-NMNO@SiO2 alone. In contrast, after incubation and irradiation with the addition of Cy-NMNO@SiO2, significant fluorescence appeared in the cells, and the fluorescence signal became stronger and stronger. The mean fluorescence intensity was shown in Fig. 2d. These results indicated that the nanoplatform was able to deliver the probe into tumor cells and activate it under NIR laser irradiation. We further validated the obtained results by flow cytometry analysis Fig. 2c.
Inspired by the well-characterized cascade reaction of Cy-NMNO@SiO2 in solution, in vitro assays were performed to visualize the intracellular NO and 1O2 production in Cy-NMNO@SiO2 cells by laser confocal microscopy using DAF-FM (a commercial NO probe) and single-linear-state oxygen green sensor (SOSG) fluorescent probes, respectively, to visualize the Cy- NMNO@SiO2 intracellular 1O2/NO production. As a result, both the NIR group alone and the cells treated with Cy-NMNO@SiO2 alone showed fluorescence intensities similar to the background signal (Fig. 2b). However, in the Cy-NMNO@SiO2 + NIR group had enhanced fluorescence, which indicated that the probe was effectively released to produce 1O2 with NO under 808 nm near-infrared laser irradiation, and 1O2 was produced by photosensitizer under laser irradiation. In addition, it has been shown in the literature that NO can react with superoxide anions in the tumor environment to form the oxidant peroxynitrite (ONOO–), which stimulates the production of matrix metalloproteinases (MMPs) in the tumor mesenchyme, thereby degrading virtually all of the collagenous components of the ECM and facilitating the penetration of therapeutic agents at the tumor site [25]. Therefore, we used TPNIR-FP detection to show that ONOO– was indeed produced during the process. These results indicate that the photoactivation of Cy-NMNO@SiO2 in living cells is successful and produces potent class I and class II responses due to the photorelease of ·NO, providing the basis for a synergistic anti-hypoxic photodynamic effect. The above results indicate that the fracture response properties of the probe can specifically respond to NIR light, and that fracture produces both photosensitizer and NO, and in the process, additionally generates ONOO–, which enhances the phototoxicity of Cy. Meanwhile, we quantitatively analyzed the fluorescence intensity in Fig. 2d and 2e using ImageJ software.1O2, NO and ONOO– were all significantly enhanced under laser irradiation. Mean fluorescence intensity (Mean) = Sum of fluorescence intensities in the area (IntDen) / Area of the region (Area).
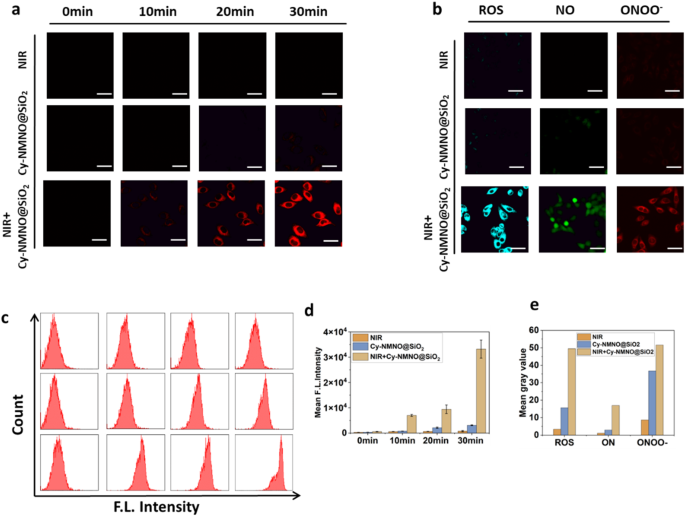
(a) Photographs of fluorescence brightness at different incubation times under laser confocal microscopy for the NIR group, Cy-NMNO@SiO2 group, and the NIR + Cy-NMNO@SiO2 group. Scale bar = 20 μm. (b) ROS, NO and ONOO– production in A375 cells were detected by SOSG, DAF-FM, TPNIR-FP probes, respectively. Scale bar = 20 μm. (c) The flow cytometry analysis of (a). (d) The mean gray value of (a). (e) The mean gray value of (b). Mean fluorescence intensity (Mean) = Sum of fluorescence intensities in the area (IntDen) / Area of the region (Area)
According to the above experimental results, Cy-NMNO showed excellent NO and 1O2 production performance. Theoretical calculations were used to reveal the effect of the molecular structure of Cy-NMNO on the NO and 1O2 production. The ability of NO to dissociate from Cy-NMNO under NIR irradiation determines the NO content. The dissociation ability can be evaluated by the bond dissociation energy (BDE) of N-NO. The bond dissociation energy (BDE) for Cy-NMNO was calculated as 32.8 kcal/mol (Figure S2). The photon energy of 808 nm infrared light is 35.4 kcal/mol, which can realize N-NO bond breaking of Cy-NMNO under NIR irradiation. In addition, we also calculated the N-NO BDE of the molecule reported in the literature. The results showed that the value of BDE is closely related to the molecular structure (Figure S2). Therefore, screening suitable photosensitizers can regulate the NO release performance by affecting BDE of N-NO bond, which provides a reference for the synthesis of complex.
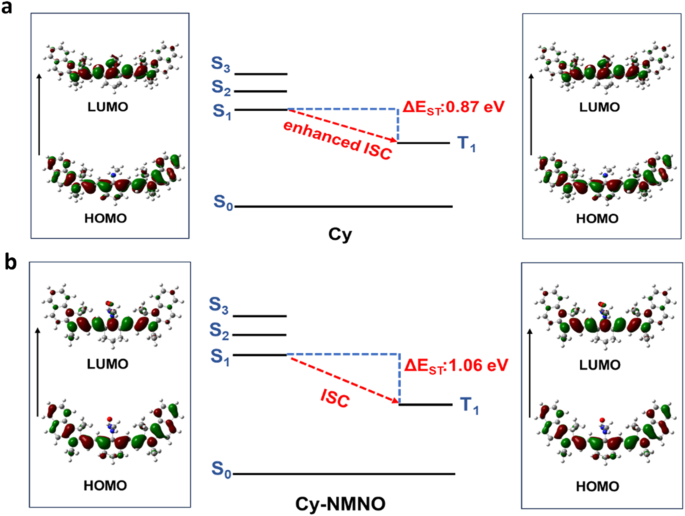
Selected frontier molecular orbitals involved in excitation and singlet/triplet excited states of Cy (a), and Cy-NMNO (b)
The N-NO bond of the Cy-NMNO breaks under NIR light irradiation, releasing the small molecule photosensitizer Cy. The performance difference between Cy-NMNO and Cy for 1O2 generation will affect the PDT performance. Efficient intersystem crossing (ISC) offers the opportunity to improve the triplet excited state yield, which is highly desirable for efficient 1O2 generation [26]. The small ΔEST (the energy gap between singlet excited state and triplet excited state) is favor to improve the ISC process. TDDFT was used to investigate the ΔEST differences between Cy and Cy-NMNO [27]. The ΔEST value of Cy and Cy-NMNO were 0.87 eV and 1.06 eV, respectively (Fig. 3a and b). From the above investigations, Cy produced by releasing NO from Cy-NMNO exhibited smaller ΔEST, which is more conducive to promoting the ISC process and 1O2 generation. The excellent 1O2 yield improved the PDT performance.
Cytotoxicity assay and photoinduced cytotoxicity
The magnitude of cytotoxicity of nanoplatforms determines whether they can be applied in the clinic or not, therefore, we examined the cytotoxicity under non-laser irradiation using Cell Counting Kit-8 (CCK-8 kit). First, we divided the cells into three groups, control, Cy@SiO2 group, and Cy-NMNO@SiO2 group. The Control group was left untreated, and the Cy@SiO2, Cy-NMNO@SiO2 groups were added with 0, 2, 4, 6, 8, 10 µg/mL of Cy@SiO2, Cy-NMNO@SiO2, respectively. As shown in Fig. 4a, after incubation of A375 cells with Cy-NMNO@SiO2 at a concentration of 10 µg/mL for 24 h, more than 85% of the cells survived. This proves that our synthesized nanoplatform Cy-NMNO@SiO2 has low cytotoxicity and can be applied to cell viability. After confirming the good in vitro biosafety of Cy-NMNO@SiO2, we proceeded to evaluate its tumor cell killing ability. As can be seen in Fig. 4b, the cell survival rate of the control group did not significantly decrease under 808 nm laser irradiation (1.5 W/cm2, 5 min). The cytotoxicity of Cy@SiO2, Cy-NMNO@SiO2 showed a concentration-dependent pattern, and as the concentration of Cy@SiO2, Cy-NMNO@SiO2 (0–10 µg/mL) increased, the survival rate of the cells gradually decreased, and the cell survival rate was almost zero when the Cy-NMNO concentration was 10 µg/mL. And it can be seen from the figure that the phototoxicity of Cy-NMNO@SiO2 was significantly higher than that of Cy@SiO2 group. This confirms our speculation that under NIR laser irradiation, the chemical bond between Cy-NMNO is broken, producing both Cy and NO. Cy has a photodynamic therapeutic effect, and nitric oxide (NO) binds to the oxygen-binding site of mitochondria, inhibiting cellular respiration and reducing endogenous O2 consumption, and also synergistically enhances the photodynamic therapy efficacy. NO can react with superoxide in the tumor environment to form oxidant peroxynitrite (ONOO–) and synergistically enhance the anti-hypoxic photodynamic effect. NO can react with superoxide anions in the tumor environment to form the oxidant peroxynitrite (ONOO–), which synergistically enhances the anti-hypoxic photodynamic effect.
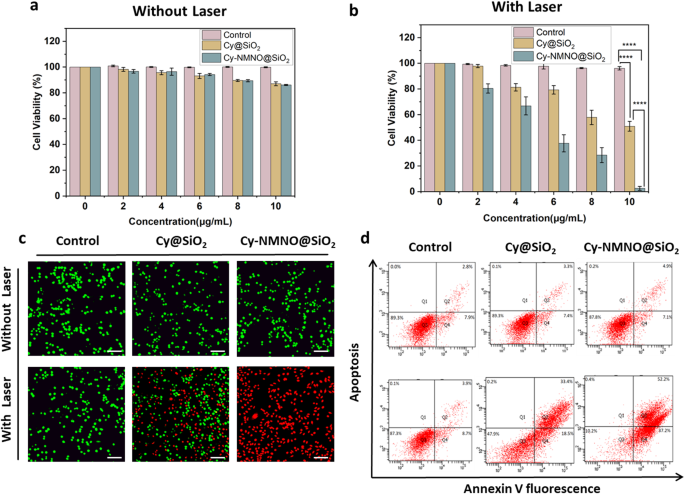
(a) (b) The cytotoxicity of A375 cells treated with PBS, Cy@SiO2, Cy-NMNO@SiO2 under laser irradiation and without laser irradiation (****P < 0.0001). (c) Calcein AM/PI co-staining imaging of A375 cells treated with PBS, Cy@SiO2, Cy-NMNO@SiO2 under laser irradiation and without laser irradiation was observed using laser scanning confocal microscopy. Scale bar = 200 μm. (d) Apoptosis was analyzed using flow cytometry under laser irradiation and without laser irradiation in control, Cy@SiO2, Cy-NMNO@SiO2 (808 nm, 1.5 W/cm2, and 5 min)
Next, we continue to confirm our speculation by Calcein AM/PI Cell Activity and Cytotoxicity Assay Kit, Annexin V-FITC Apoptosis Kit, Western blot, etc. Calcein/PI Cell Activity and Cytotoxicity Assay Kit is a very convenient kit for the detection of dead and alive animal cells based on double fluorescence staining method of Calcein-AM (calcineurin) and PI (Propidium Iodide) dual fluorescence staining method to detect the dead and alive animal cells, which is based on the principle that the two probes detect the intracellular esterase activity and cell membrane integrity, respectively, and thus respond to the cell’s live and dead status. Calcein AM stained live cells in green, while Propidium Iodide (PI) stained dead cells in red. As can be seen from the Fig. 4c, in the absence of laser irradiation, all A375 cells were green and not dead. While some cells in the Cy@SiO2 group died under laser irradiation, almost all cells in the Cy-NMNO@SiO2 group died. This is consistent with the results of our CCK-8 assay, which showed that the phototoxicity of Cy-NMNO@SiO2 was significantly higher than that of Cy@SiO2 group. In addition, we also verified the results with Annexin V-FITC apoptosis kit and statistically analyzed to obtain that the apoptosis rate of Cy-NMNO@SiO2 was significantly higher than that of Cy@SiO2 group (Fig. 4d, S5) (P < 0.0001).
It is well known that the microenvironment of many solid tumors is hypoxic. In particular, the constant consumption of oxygen to generate cell-damaging ROS during PDT further exacerbates tumor hypoxia, severely impeding the generation of efficient ROS and leading to suboptimal PDT efficiency [28]. Hypoxia-inducible factor-1α (HIF-1α) is a nuclear transcription factor produced by cells in response to hypoxia and is a major member of the hypoxia-inducible factor family. It is normally located in the cytoplasm, and the α-subunit is rapidly ubiquitinated and degraded when the intracellular oxygen partial pressure is normal. However, under hypoxia, HIF-1α accumulates, translocates from the cytoplasm to the nucleus and binds to the β-subunit, initiating a series of genes that contribute to the adaptation of cells to hypoxia, and HIF-1α is the major hypoxia-associated gene [29,30,31]. To verify the hypoxia tolerance of Cy-NMNO@SiO2, the expression of HIF-1α was detected by Western blot after Cy-NMNO@SiO2 was applied to A375 cells with different irradiation times. As shown in Fig. 5a, b, c, d and e, the cell-related apoptotic proteins Bax, Caspase-9, Cytochrome C increased with irradiation time, which proved that our nanoplatform Cy-NMNO@SiO2 did have a therapeutic effect on PDT. However, the expression of HIF-1α decreased with irradiation time. This suggests that our nanoplatform Cy-NMNO@SiO2 can ultimately generate NO under NIR laser irradiation. NO, as an important vasotension-regulating messenger, can achieve the alleviation of tumor hypoxia [32].
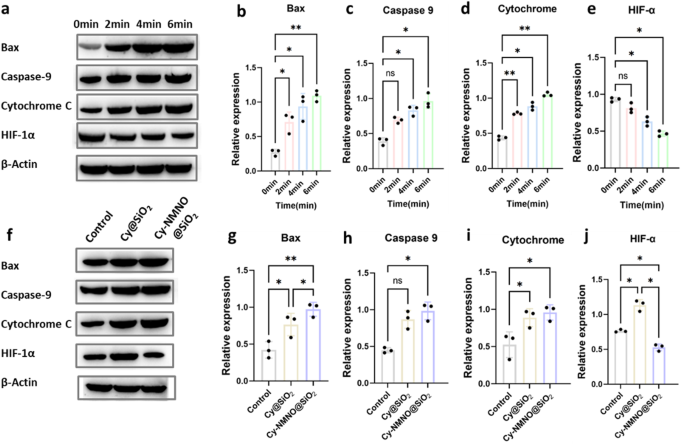
(a) Protein expression of Bax, Caspase-9, Cytochrome C, HIF-1α of A375 cells treated with PBS, Cy@SiO2, Cy-NMNO@SiO2 were laser at different times using western blot analysis and using the expression of β-actin as a control. (b) (c) (d) (e) Quantitative analysis of Bax, Caspase-9, Cytochrome C, HIF-1α expression in (a). (f) Protein expression of Bax, Caspase-9, cytochrome C, and HIF-1α in A375 tumor-bearing mice with various treatments, and β-actin expression was used as a control. (g) (h) (i) (j) Quantitative analysis of Bax, Caspase-9, Cytochrome C, HIF-1α expression in (f)
To reconfirm our speculation, we injected different therapeutic drugs Cy@SiO2, Cy-NMNO@SiO2 intravenously into the homozygous BALB/c mice using PBS as a control, respectively. As shown in the Fig. 5f, g, h, i and j, the apoptotic protein expression of Cy-NMNO@SiO2 group was significantly higher than that of the control group and the Cy@SiO2 group (P < 0.05), but the expression of HIF-1α was significantly lower than the control and Cy@SiO2 groups (P < 0.05). These results above suggest that Cy-NMNO@SiO2 on the one hand can generate sufficient ROS to significantly amplify oxidative stress in vivo for tumor PDT therapy. However, in the case of PDT alone, the treatment exacerbates the hypoxic environment at the tumor site, whereas the precisely controlled release of NO at the tumor site helps to alleviate the hypoxia at the tumor site and synergistically enhances the effect of PDT treatment. The above experiments confirm that our nanoplatform can indeed achieve the treatment of skin cancer and improve the high hypoxia at the tumor site due to the consumption of oxygen by PDT.
Antimicrobial testing
Current clinical strategies to combat bacteria focus on antibiotic therapy. However, these strategies face increasing challenges due to the development of drug-resistant bacteria. The era of antibiotic drugs as the mainstay against harmful bacteria is reportedly over. Therefore, in order to protect public health, there is an urgent need for research and development of innovative antimicrobial agents with high antimicrobial activity and without inducing bacterial resistance. Photodynamic therapy (PDT)-based antimicrobial agents may be a promising alternative.
Next, we validated the antibacterial effect of the nanoplatform Cy-NMNO@SiO2. The dilution plate method was used to evaluate the synergistic PDT/NO bactericidal effect of Cy-NMNO@SiO2 against Gram-positive bacteria (Staphylococcus aureus). Staphylococcus aureus was inoculated in liquid medium of Luria-Bertani (LB) and incubated overnight at 37 °C in an incubator with shaking. Staphylococcus aureus was randomly divided into three groups, control group, Cy@SiO2 group and Cy-NMNO@SiO2 group, and according to their groupings, the same concentration of PBS, Cy@SiO2, Cy-NMNO@SiO2 were added and irradiated (808 nm, 1.5 W/cm2, 5 min) with and without irradiation, respectively. As shown in Fig. 6a, the number of colonies in the Cy@SiO2 + NIR-treated group was significantly reduced compared with the control, Cy@SiO2 group and Cy-NMNO@SiO2 without 808 nm laser irradiation. The inactivation rate reached 36.4% in the Cy@SiO2 + NIR group (Fig. 6b). Cy, as a photosensitizer, can produce reactive oxygen species, which has a therapeutic effect on photochemotherapy. In the Cy-NMNO@SiO2 + NIR group, only a small amount of bacteria survived, and the inactivation rate was as high as 94.4%.
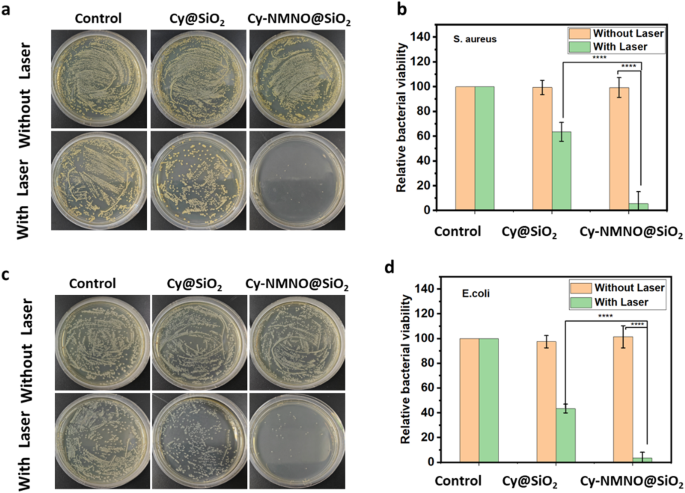
Photographs of bacterial colonies formed by (a) S. aureus (c) E. coli based on plate count method. The corresponding bacterial viabilities of b) S. aureus d) E. coli. Treated with PBS, Cy-Cy@SiO2, Cy-NMNO@SiO2 without or with 808 nm laser irradiation (1.5 W cm− 2, 5 min) (****P < 0.0001)
This indicates that NO was released from the photosensitizer during laser irradiation. NO is a typical lipophilic biosignaling molecule, a broad-spectrum antimicrobial candidate, and its antimicrobial process does not depend on the type of bacteria compared with traditional antibiotics. NO is able to produce additional ONOO–, RNS, etc. in bacteria and the by-products of NO are able to disrupt bacterial cell membranes due to nitrosation and oxidative stress [33]. It also induces significant DNA damage, which makes the bacteria more susceptible to damage and thus has a bactericidal effect. Gram-negative bacteria have an additional membrane layer in their cell wall structure compared to Gram-positive bacteria, which makes it often difficult for typical PDT agents to penetrate these bacteria. However, our small molecule photosensitizer Cy has a positive charge and bacteria have a negative charge, which can enhance the interaction between the photosensitizer and the bacterial outer membrane and improve its PDT antimicrobial properties. Therefore, we similarly tested the antimicrobial effect of Cy-NMNO@SiO2 against E. coli (Gram-negative bacteria). As shown in Fig. 6c, the bacterial growth was normal without NIR laser irradiation, but only a small amount of bacteria survived in the Cy-NMNO@SiO2 + NIR group, with an inactivation rate of 96.6% (Fig. 6d). This proves that the Cy-NMNO@SiO2 also has a good antibacterial effect on Gram-negative bacteria under NIR light irradiation.
In vivo imaging and anti-tumor therapy in tumor-bearing mice
In vivo bioimaging plays a crucial role in the accurate diagnosis and treatment of tumors. Since Cy-NMNO can have “off-on” fluorescence activation under near-infrared laser irradiation, we utilized this property to study the in vivo activation of Cy-NMNO@SiO2 in mice. When the tumor volume of A375 ruffed mice increased to about 150 mm3, Cy-NMNO@SiO2 was injected through the tail vein. The fluorescence signal at the tumor site of ruffed mice was gradually enhanced after 808 nm laser irradiation. We euthanized the mice at 0 h, 6 h, 12 h, 24 h and 36 h, respectively, and quantitatively analyzed the fluorescence intensity of each group by ImageJ software after being photographed by a fluorescence imaging system. The schematic illustration of treatment route was shown in Fig. 7a. As shown in Fig. 7b, the fluorescence of the tumor tissue appeared 6 h after the intravenous injection of Cy-NMNO@SiO2, reached the maximum intensity at 12 h, and then gradually weakened. These data indicate that Cy-NMNO@SiO2 can be activated under near-infrared light irradiation to produce Cy and realize fluorescence imaging.
In order to study the therapeutic effect of Cy-NMNO@SiO2 on the mouse skin melanoma model, when the volume tumor volume of A375 loaded mice reached about 150 mm3, we randomly divided the loaded mice into 6 groups: PBS group, PBS + Laser group, Cy@SiO2 group, Cy@SiO2 + Laser, Cy-NMNO@SiO2, and Cy- NMNO@SiO2 + Laser groups, 10 mice in each group. We recorded the body weights and tumor volumes of the mice (at 2-day intervals after irradiation) and calculated the relative tumor volumes of the mice. (Fig. 7c, d, and f). The relative tumor volumes of the PBS + Laser group both increased by about 5.5-fold, indicating that single laser irradiation had little effect on tumor growth.
Although the Cy@SiO2 + Laser group had a certain inhibitory effect on the tumor treatment, the tumor volume was still increasing gradually, and it failed to achieve the effect of completely treating the tumor. Notably, the Cy-NMNO@SiO2 + NIR group showed significant tumor inhibition with no regrowth. Meanwhile, combined with the results of Western blot above, Cy-NMNO was shown to be an effective synergistic PDT-GT tumor therapeutic agent. In order to further observe the killing ability of Cy-NMNO@SiO2 on tumor tissues after treatment to verify its anti-tumor effect. We observed the status of tumor tissues by hematoxylin & eosin (H&E) staining (Fig. 7e). H&E staining showed that the tumor tissue damage in Cy-NMNO@SiO2 + Laser group was obvious, and the efficacy was significant. While PBS, PBS + Laser, Cy@SiO2 group, and Cy-NMNO@SiO2 did not show significant tumor damage. The Cy@SiO2 group + Laser group had some therapeutic effect on the tumor, but the therapeutic effect was limited. Meanwhile, in order to explore the in vivo safety during antitumor treatment, the weight of the hormonal mice was recorded during the tumor suppression experiments in hormonal mice, and the mice were weighed every other day. And the survival rate of mice was calculated (Fig. 7e). As shown in Fig. 7c, there was no significant change in the body weight of all mice, and the mice in the Cy-NMNO@SiO2 + Laser treatment group always survived. In order to prove the safety of the nanoplatform again, we observed the normal organs (heart, liver, spleen, lung and kidney) of the surviving mice in Cy-NMNO@SiO2 by sectioning and found that there was no obvious damage to the heart, liver, spleen, lungs, kidneys and other organs of the mice. This indicates that Cy-NMNO@SiO2 has good biosafety in the therapeutic dose range with negligible side effects. It can be seen that Cy-NMNO@SiO2 can effectively treat tumors under near-infrared laser irradiation without obvious toxic side effects.
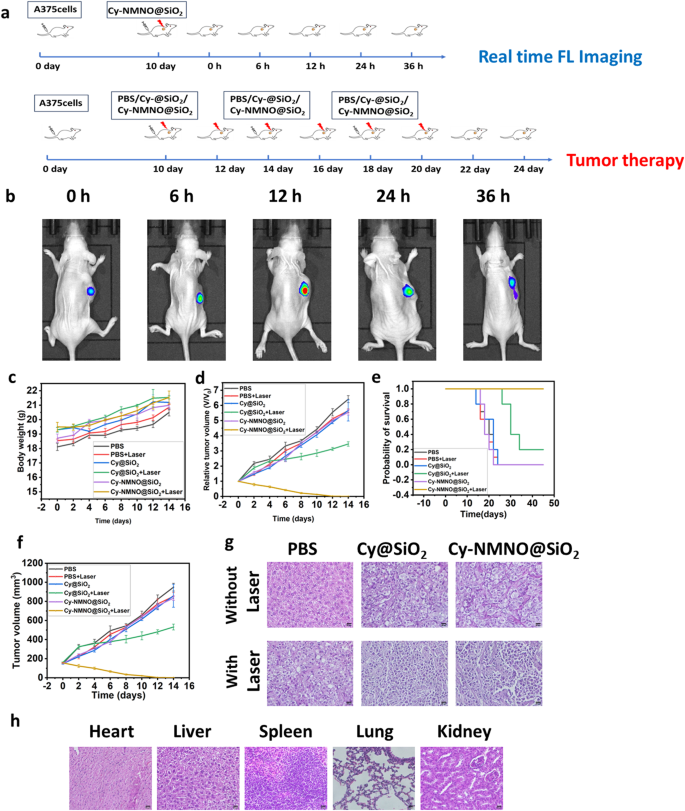
(a) Schematic illustration of treatment route. (b) Real-time fluorescence imaging in A375 tumor-bearing mice (c) Body weight change, (d) Relative tumor volume, (e) Probability of survival, (f) Tumor volume of A375 tumor-bearing mice with various treatments. (g) H&E staining of tumor after various treatments. Scale bar = 20 μm. (h) Results of H&E staining of heart, liver, spleen, lung and kidney of surviving mice in the Cy-NMNO@SiO2 + laser treatment group. Scale bar = 20 μm